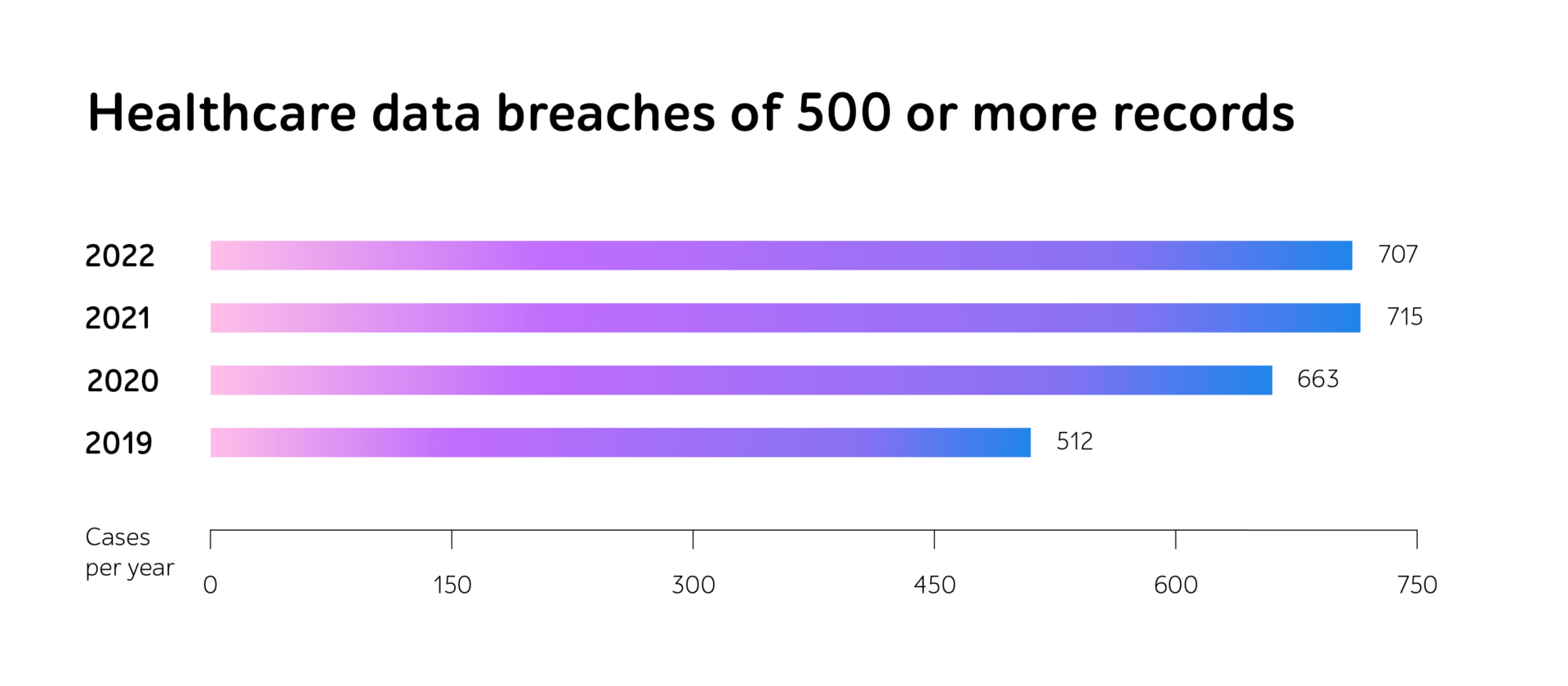
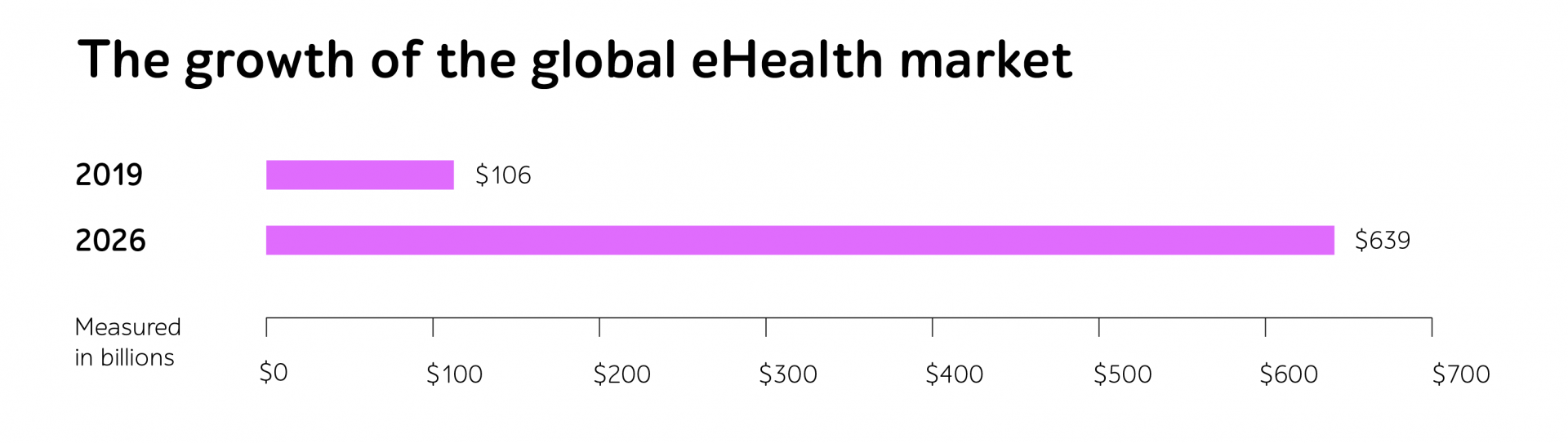
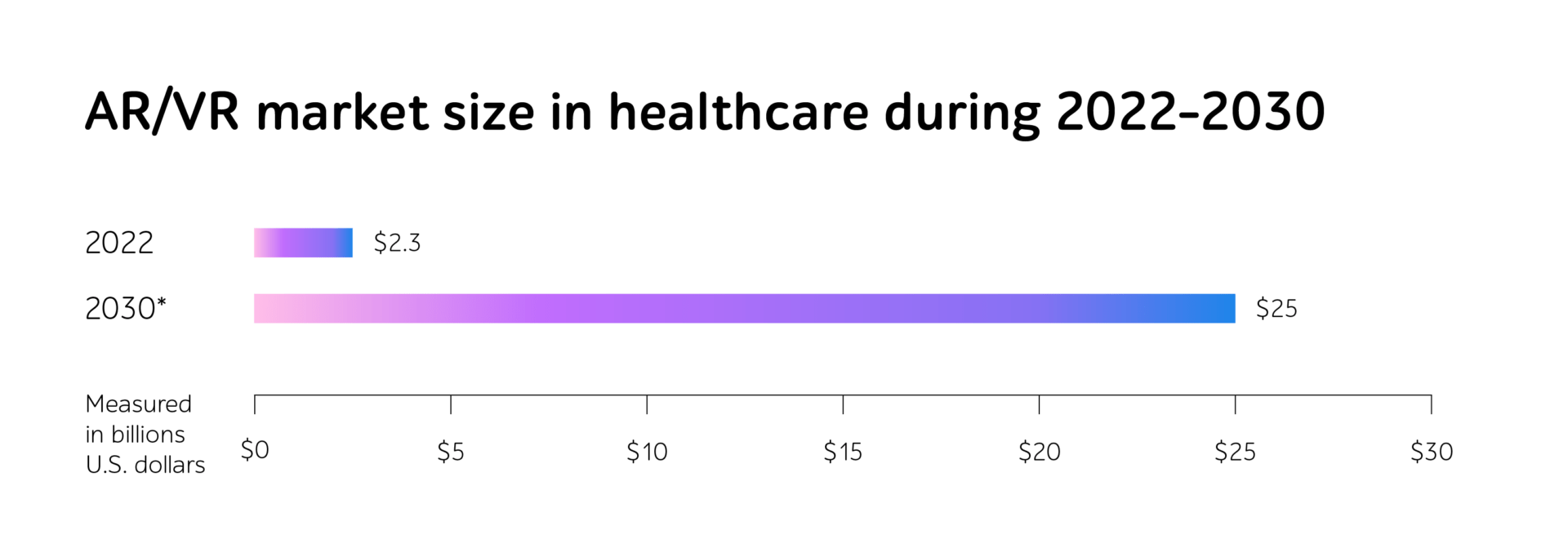Whether companies develop dedicated systems to gather and process session data from blood transfusion devices, connected insulin pumps, smart inhalers, home dialysis machines, or any other intricate eHealth IT products, the journey from a concept to a fully reliable medical device involves numerous phases of testing.
In addition to multiple laboratory validations, equally important is the involvement of seasoned QA engineers who ensure that embedded medical software intended for these devices meets the required operational and security standards. Moreover, they help confirm that artifacts correspond to the demands of regulators like FDA and align with standards such as IEC 62304 and ISO 14971.
In this article, let’s delve into QA’s role in creating failsafe electronic healthcare products and essential software testing activities for ensuring compliance, reliability, and patient safety.
Why correctly functioning embedded software matters within medical devices
Medical devices have progressed from simple mechanical instruments to sophisticated, software-driven systems. Embedded code now handles everything from sensor calibration and drug delivery to touchscreen controls and secure wireless data exchange. Modern MRI scanners, laser-surgery units, anesthesia machines, PET scanners, ventilators, and many other devices depend on this software to operate safely and accurately.
Within this context, even a minor glitch can have life-threatening consequences. While software errors in consumer products may lead to just inconvenience, in medical devices they present serious risks to patient safety, underscoring the need for rigorous, fail-safe performance. Just have a look.
Several years ago, critical vulnerabilities were detected in insulin pumps that could enable attackers to remotely interfere and set up incorrect doses of insulin. Although the FDA issued a safety notice, the manufacturer didn’t act right away. To drive the point home, the researchers built a proof-of-concept exploit; the public exposure finally forced a product recall. Or another example. A company recalled its software following the discovery of a critical defect that could cause mismatches between programmed drug data and the infusion device it supports. This posed a risk of incorrect medication dosing, leading to serious or even fatal outcomes.
Moreover, eHealth is a highly regulated industry, where even the slightest incompliance with international standards such as IEC 62304 can trigger serious legal and financial repercussions, including fines, exclusion from key markets like the EU or US, and disruptions to product timelines. The ripple effect also incorporates diminished trust from customers and stakeholders. That’s why rigorous software compliance isn’t only a regulatory necessity but a cornerstone of sustainable business strategy.
End-to-end QA strategy to support device manufacturers and healthcare institutions
Early and rigorous embedded software testing is crucial to keep medical devices reliable under every intended condition. Here’s the list of vital QA activities reinforcing eHealth device development and addressing potential hazards caused by software errors.
- Performance testing
This activity is critically important when ensuring quality of software embedded in medical devices, as they often operate in life-critical environments where failure or underperformance can lead to serious harm or even death. By rigorously evaluating the system’s behavior under both typical and extreme conditions, this process helps prevent unexpected failures and ensure dependable operation.
During testing, QA engineers design test scenarios that closely mimic real-world operating conditions, simulating sensors operating simultaneously, user interactions, and network communications. Afterward, they run stress, load, spike, soak testing, and other vital verifications to confirm the device can seamlessly handle the load during set time without any degradation, memory leaks, or resource exhaustion.
Additionally, often the network of systems under test and medical devices is very intricate, requiring QA engineers to come up with tailored performance testing approaches. For instance, for a company that created a system for processing session data from blood transfusion devices, QA engineers created emulators and complex data simulations to replicate behavior of live medical equipment under various load conditions. This enabled the team to evaluate how the software handled high-frequency data streams, device synchronization, and real-time processing demands within a multi-hospital environment.
- Functional testing
Functional testing of embedded medical device software confirms that it consistently performs as intended under all conditions, adheres to clinical requirements, and assists medical practitioners in making accurate decisions.
To ensure failsafe operation of every build, QA specialists simulate real-world scenarios and perform various verifications (from smoke and new feature testing to regression checks and defect validation) to validate key functions, such as data input/output or device responses. This approach helps maintain software stability throughout the entire development life cycle and prevent issues from slipping into the production environment.
- Security testing
Security testing is non-negotiable for embedded medical-device software: a single vulnerability can let attackers manipulate treatment settings, steal patient data, or knock out hospital systems. Assessments must therefore follow strict standards including FDA guidance, HIPAA, IEC 62304, and similar rules, to guarantee data privacy and to ensure that no failure can jeopardize patient safety or lead to fatal outcomes.
During a comprehensive audit intended to gauge software security level, QA engineers conduct penetration testing replicating the attacks performed by malicious intruders to analyze the system’s ability to resist and remain operable despite anything. With the help of static code analysis, they can early catch such code-related flaws as injections or unsafe functions, assisting developers in mitigating problems before the deployment. Testing experts also confirm that QA artifacts (test plans, test cases, defect reports, risk assessments, traceability matrices, etc.) follow regulatory requirements, ensuring that all detected security problems have been properly addressed.
- Localization testing
Medical device software must be precisely tailored for each market it serves. Localization testing helps address linguistic, cultural, and legal variations, supporting both safety and usability. QA engineers lead this effort by validating that the interface and functionality remain consistent and compliant in all supported locales.
To be precise, they make sure the whole text is translated and is in line with medical language, fields accept localized characters, error messages, warnings, speech output work correctly in necessary languages, date and time are of suitable formats, the interface adjusts seamlessly to changed text lengths, and other vital aspects.
- Compliance testing
Ensuring regulatory compliance is essential when developing embedded software for medical devices, as these systems directly influence patient well-being. This process evaluates whether the embedded software can withstand unexpected scenarios and has necessary safety measures or failure responses. It also ensures that every requirement is validated through test cases, with detailed documentation maintained for regulatory audits.
During this crucial step, QA engineers analyze requirements to construct test coverage matrices that ensure complete verification. They create strategic test plans that meet regulatory guidelines, run tests, and confirm that potential software risks are properly mitigated. Their work culminates in the preparation of audit-ready artifacts to help companies pass regulatory review.
- Test automation
Market urgency pushes teams to release feature-rich IT solutions rapidly, often leaving insufficient time for thorough testing. This can turn into a particularly pressing issue if organizations mainly rely on manual verifications, as they risk facing prolonged testing workflows that will only escalate with each additional software module.
That’s why test automation can become a valuable addition to the project, allowing specialists to meet quality gates on time, which is proved by 60% of the latest World Quality Report respondents who stated that test automation helped them successfully deliver more features.
During the project, QA automation engineers can configure CI/CD pipelines, create tailored AI-empowered test automation frameworks from scratch if necessary, develop scripts to cover extensive regression and new feature testing scope, and ensure ongoing maintenance of the established solution.
Failure isn’t an option
Within an eHealth industry, people’s lives often depend on tech innovations. Medical devices levering them should operate flawlessly without compromising safety, accuracy, or regulatory integrity. Comprehensive QA plays a pivotal role in ensuring failsafe operation of intricate software embedded in this equipment, helping gain regulatory approval and eliminate even minor glitches.
Want to ensure regulatory readiness of your medical device embedded software and prevent operational disruptions? Contact a1qa’s team to get a professional consultation.